Androgen backdoor pathway
The androgen backdoor pathway is a collective name for all metabolic pathways where clinically relevant androgens are synthesized from 21-carbon steroids (pregnanes) by their 5α-reduction with roundabout of testosterone and/or androstenedione.[1] Initially described as pathway where 5α-reduction of 17α-hydroxyprogesterone ultimately leads to 5α-dihydrotestosterone,[2] several other pathways have been since then discovered that lead to 11-oxygenated androgens which are potent agonists of the androgen receptors.[3] A backdoor pathway is an alternative to the conventional,[4] canonical[5] androgenic pathway that involves testosterone and/or androstenedione.
Introduction
The androgen backdoor pathways are critical metabolic processes involved in the synthesis of clinically relevant androgens from 21-carbon steroids (pregnanes) through their 5α-reduction. These pathways occur without the involvement of testosterone and/or androstenedione, which are part of the conventional, canonical androgenic pathway. Initially, the 5α-reduction of 17α-hydroxyprogesterone was described in medical literature[2] as a pathway that ultimately leads to the production of 5α-dihydrotestosterone. However, over the last two decades, several other distinct pathways have been discovered that lead to the synthesis of 11-oxygenated androgens, which are potent agonists of the androgen receptors. The androgen response mechanism occurs through the binding of androgens to cytosolic androgen receptors, which are translocated to the nucleus upon androgen binding and ultimately regulate gene transcription via androgen responsive elements. This response mechanism plays a crucial role in male sexual differentiation and puberty, as well as other tissue types and processes. The discovery of the backdoor pathway to 5α-dihydrotestosterone in the tammar wallaby in 2003[6] opened new avenues for understanding the biosynthesis of androgens in humans. Subsequently, other backdoor pathways leading to potent 11-oxygenated androgens have also been characterized,[7] providing further insight into the synthesis of androgens in vivo. Understanding these pathways is critical for the development of effective treatments for conditions related to androgen biosynthesis.[1]
Biochemistry
Dihydrotestosterone backdoor biosynthesis
The primary feature of the androgen backdoor pathway is that 17α-hydroxyprogesterone (17-OHP) can be 5α-reduced and finally converted to 5α-dihydrotestosterone (DHT) via an alternative route that bypasses the conventional[4] intermediates androstenedione and testosterone.[2][9]
This route is activated during normal prenatal development and leads to early male sexual differentiation.[10][11][12] It was first described in the marsupials and later confirmed in humans.[13] Both the canonical and backdoor pathways of DHT biosynthesis are required for normal human male genital development, thus defects in the backdoor pathway from 17-OHP or progesterone (P4) to DHT lead to undervirilization in male fetuses because placental P4 is the precursor of DHT via the backdoor pathway.[1]
In 21-hydroxylase deficiency[9] or cytochrome P450 oxidoreductase deficiency,[14] this route may be activated regardless of age and sex by even a mild increase in circulating 17-OHP levels.[15]
While 5α-reduction is the last transformation in the classical androgen pathway, it is the first step in the backdoor pathways to 5α-dihydrotestosterone that acts on either 17-OHP or P4 which are ultimately converted to DHT.[1]
17α-Hydroxyprogesterone pathway
The first step of this pathway is the 5α-reduction of 17-OHP to 5α-pregnan-17α-ol-3,20-dione (17OHDHP, since it is also known as 17α-hydroxy-dihydroprogesterone). The reaction is catalyzed by SRD5A1.[16][14] 17OHDHP is then converted to 5α-pregnane-3α,17α-diol-20-one (5α-Pdiol) via 3α-reduction by a 3α-hydroxysteroid dehydrogenase isozyme (AKR1C2 and AKR1C4)[5][13] or HSD17B6, that also has 3α-reduction activity.[17][18] 5α-Pdiol is also known as 17α-hydroxyallopregnanolone or 17OH-allopregnanolone. 5α-Pdiol is then converted to 5α-androstan-3α-ol-17-one, also known as androsterone (AST) by 17,20-lyase activity of CYP17A1 which cleaves a side-chain (C17-C20 bond) from the steroid nucleus, converting a C21 steroid (a pregnane) to C19 steroid (an androstane or androgen). AST is 17β-reduced to 5α-androstane-3α,17β-diol (3α-diol) by HSD17B3 or AKR1C3.[19] The final step is 3α-oxidation of 3α-diol in target tissues to DHT by an enzyme that has 3α-hydroxysteroid oxidase activity, such as AKR1C2,[20] HSD17B6, HSD17B10, RDH16, RDH5, and DHRS9.[14] This oxidation is not required in the classical androgen pathway.[1] The pathway can be summarized as: 17-OHP → 17OHDHP → 5α-Pdiol → AST → 3α-diol → DHT.[1]
Progesterone pathway
The pathway from P4 to DHT is similar to that described above from 17-OHP to DHT, but the initial substrate for 5α-reductase here is P4 rather than 17-OHP. Placental P4 in the male fetus is the feedstock for the backdoor pathway found operating in multiple non-gonadal tissues.[5] The first step in this pathway is 5α-reduction of P4 towards 5α-dihydroprogesterone (5α-DHP) by SRD5A1. 5α-DHP is then converted to allopregnanolone (AlloP5) via 3α-reduction by AKR1C2 or AKR1C4. AlloP5 is then converted to 5α-Pdiol by the 17α-hydroxylase activity of CYP17A1. The pathway then proceeds the same way as the pathway that starts from 17-OHP, and can be summarized as: P4 → 5α-DHP → AlloP5 → 5α-Pdiol → AST → 3α-diol → DHT.[1]
11-Oxygenated androgen backdoor biosynthesis
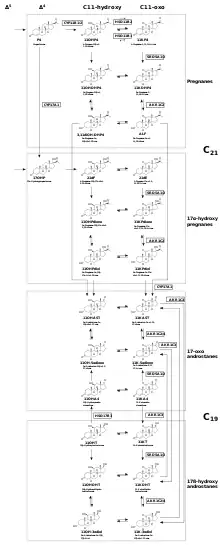
There are two known 11-oxygenated androgens, 11-ketotestosterone (11KT) and 11-ketodihydrotestosterone (11KDHT), which both bind and activate the androgen receptor with affinities, potencies, and efficacies that are similar to that of T and DHT, respectively.[1] Some work[21][22][23] suggests that though 11OHT and 11OHDT may not have significant androgenic activity as they were once thought to possess, they may still be important precursors to androgenic molecules.[1] The relative importance of the androgens depends on their activity, circulating levels and stability. The steroids 11OHA4 and 11KA4 have been established as having minimal androgen activity,[24][1][25] but remain important molecules in this context since they act as androgen precursors.
The backdoor pathways to 11-oxygenated androgens can be broadly as two Δ4 steroid entry points (17-OHP and P4) that can undergo a common sequence of three transformations:
- 11β-hydroxylation by CYP11B1 in the adrenal cortex,
- 5α-reduction by SRD5A1/2,
- reversible 3α-reduction/oxidation of the ketone/alcohol by AKR1C2 or AKR1C4.
Clinical significance
In CAH due to deficiency of 21-hydroxylase[9] or cytochrome P450 oxidoreductase (POR),[14][26] the associated elevated 17-OHP levels result in flux through the backdoor pathway to DHT that begins with 5α-reduction of 17-OHP . This pathway may be activated regardless of age and sex.[27] Fetal excess of 17-OHP in CAH may contribute to DHT synthesis that leads to external genital virilization in newborn girls with CAH.[14] P4 levels may also be elevated in CAH,[28][29] leading to androgen excess via the backdoor pathway from P4 to DHT.[30] 17-OHP and P4 may also serve as substrates to 11-oxygenated androgens in CAH.[31]
Serum levels of the C21 11-oxygenated steroids 11OHP4 and 21dF have been known to be elevated in (non-classical/classical) CAH since about 1990,[32][33] and LC-MS/MS profiles that include these steroids have been proposed for clinical applications.[34] Classical CAH patients receiving glucocorticoid therapy had C19 11-oxygenated steroid serum levels that were elevated 3-4 fold compared to healthy controls.[35] In that same study, the levels of C19 11-oxygenated androgens correlated positively with conventional androgens in women but negatively in men. The levels of 11KT were 4 times higher compared to that of T in women with the condition. In adult women with CAH, the ratio of DHT produced in a backdoor pathway to that produced in a conventional pathway increases as control of androgen excess by glucocorticoid therapy deteriorates.[36] In CAH patients with poor disease control, 11-oxygenated androgens remain elevated for longer than 17-OHP , thus serving as a better biomarker for the effectiveness of the disease control.[37][38] In males with CAH, 11-oxygenated androgen levels may indicate the presence testicular adrenal rest tumors.[38][39] Both the classical and backdoor androgen pathway to DHT are required for normal human male genital development.[26][10] Deficiencies in the backdoor pathway to DHT from 17-OHP or from P4[13][16] lead to underverilization of the male fetus,[40][41] as placental P4 is a precursor to DHT in the backdoor pathway.[5]
A case study[13] of five 46,XY (male) patients from two families demonstrated that atypical genital appearance were attributed to mutations in AKR1C2 and/or AKR1C4, which operate exclusively in the backdoor pathway to DHT. Mutations in the AKR1C3 and genes involved in the classical androgen pathway were excluded as the causes for the atypical appearance. The 46,XX (female) relatives of affected patients, having the same mutations, were phenotypically normal and fertile. Although both AKR1C2 and AKR1C4 are needed for DHT synthesis in a backdoor pathway, the study found that mutations in AKR1C2 only were sufficient for disruption.[13] However, these AKR1C2/AKR1C4 variants leading to DSD are rare and have been only so far reported in just those two families.[42] This case study emphasizes the role of AKR1C2/4 in the alternative androgen pathways.
Isolated 17,20-lyase deficiency syndrome due to variants in CYP17A1, cytochrome b5, and POR may also disrupt the backdoor pathway to DHT, as the 17,20-lyase activity of CYP17A1 is required for both classical and backdoor androgen pathways. This rare deficiency can lead to DSD in both sexes, with affected girls being asymptomatic until puberty, when they show amenorrhea.[42]
11-oxygenated androgens may play important roles in DSDs.[43][44][14] 11-oxygenated androgen fetal biosynthesis may coincide with the key stages of production of cortisol — at weeks 8–9, 13–24, and from 31 and onwards. In these stages, impaired CYP17A1 and CYP21A2 activity lead to increased ACTH due to cortisol deficiency and the accumulation of substrates for CYP11B1 in pathways to 11-oxygenated androgens and could cause abnormal female fetal development.[43]
History
In 1987, Eckstein et al.[45] incubated rat testicular microsomes in the presence of radiolabelled steroids and demonstrated that 5α-androstane-3α,17β-diol is preferentially produced from 17α-hydroxyprogesterone (17-OHP ). While "androstanediol" was used to denote both 5α-androstane-3α,17β-diol and 5α-androstane-3β,17β-diol, "3α-diol" is used here to abbreviate 5α-androstane-3α,17β-diol in this paper as it is a common convention and emphasizes it as the 3α-reduced derivative of DHT. The function of 3α-diol was not known at that time.
Tammar wallaby pouch young do not show sexually dimorphic circulating levels of T and DHT during prostate development which suggested that another androgenization mechanism was responsible. In 2000, Shaw et al.[11] demonstrated that circulating 3α-diol mediates prostate development in these pouch young via conversion to DHT in target tissues. While 3α-diol's AR binding affinity is five orders of magnitude lower than DHT (generally described as AR inactive), it was known 3α-diol can be oxidized back to DHT via the action of a number of dehydrogenases.[46]
In 2003, Wilson et al.[6] incubated the testes of tammar wallaby pouch young with radiolabelled progesterone to show that 5α-reductase expression in this tissue enabled a novel pathway from 17-OHP to 3α-diol without T as an intermediate: |17-OHP → 17OHDHP → 5α-Pdiol → AST → 3α-diol. In 2004, Mahendroo et al.[12] demonstrated that an overlapping novel pathway is operating in mouse testes, generalizing what had been demonstrated in tammar wallaby: P4 → 5α-DHP → AlloP5→ 5α-Pdiol → AST → 3α-diol. The term "backdoor pathway" was coined by Auchus in 2004[2] and defined as a route to DHT that: 1) bypasses conventional intermediates androstenedione (A4) and T; 2) involves 5α-reduction of 21-carbon (C21) pregnanes to 19-carbon (C19) androstanes; and 3) involves the 3α-oxidation of 3α-diol to DHT. The backdoor pathway explained how androgens are produced under certain normal and pathological conditions in humans when the classical androgen pathway cannot fully explain the observed consequences. The pathway Auchus defined adds DHT to the terminus of the pathway described by Wilson et al. in 2003: 17-OHP → 17OHDHP → 5α-Pdiol → AST → 3α-diol → DHT. The clinical relevance of these results was demonstrated in 2012 for the first time when Kamrath et al.[9] attributed the urinary metabolites to the androgen backdoor pathway from 17-OHP to DHT in patients with steroid 21-hydroxylase (encoded by the gene CYP21A2) enzyme deficiency. Barnard et al.[47] in 2017 demonstrated metabolic pathways from C21 steroids to 11KDHT that bypasses A4 and T in vitro in a prostate cancer derived cell line, an aspect that is similar to that of the backdoor pathway to DHT. These newly discovered pathways to 11-oxygenated androgens were also described as "backdoor" pathways due to this similarity, and were further characterized in subsequent studies.[48][31]
Note
![]() This article was submitted to WikiJournal of Medicine for external academic peer review in 2022 (reviewer reports). The updated content was reintegrated into the Wikipedia page under a CC-BY-SA-3.0 license (2023). The version of record as reviewed is:
Maxim Masiutin; Maneesh Yadav; et al. (3 April 2023). "Alternative androgen pathways" (PDF). WikiJournal of Medicine. 10 (1): 3. doi:10.15347/WJM/2023.003. ISSN 2002-4436. Wikidata Q100737840.
This article was submitted to WikiJournal of Medicine for external academic peer review in 2022 (reviewer reports). The updated content was reintegrated into the Wikipedia page under a CC-BY-SA-3.0 license (2023). The version of record as reviewed is:
Maxim Masiutin; Maneesh Yadav; et al. (3 April 2023). "Alternative androgen pathways" (PDF). WikiJournal of Medicine. 10 (1): 3. doi:10.15347/WJM/2023.003. ISSN 2002-4436. Wikidata Q100737840.
See also
References
- Masiutin, Maxim; Yadav, Maneesh (2023). "Alternative androgen pathways". WikiJournal of Medicine. 10: X. doi:10.15347/WJM/2023.003. S2CID 257943362.
- Auchus RJ (November 2004). "The backdoor pathway to dihydrotestosterone". Trends in Endocrinology and Metabolism. 15 (9): 432–8. doi:10.1016/j.tem.2004.09.004. PMID 15519890. S2CID 10631647.
- Turcu AF, Nanba AT, Auchus RJ (2018). "The Rise, Fall, and Resurrection of 11-Oxygenated Androgens in Human Physiology and Disease". Horm Res Paediatr. 89 (5): 284–291. doi:10.1159/000486036. PMC 6031471. PMID 29742491.
- Kinter, K. J.; Anekar, A. A. (2021). Biochemistry, Dihydrotestosterone. StatPearls. PMID 32491566.
- O'Shaughnessy, Peter J.; Antignac, Jean Philippe; Le Bizec, Bruno; Morvan, Marie-Line; Svechnikov, Konstantin; Söder, Olle; Savchuk, Iuliia; Monteiro, Ana; Soffientini, Ugo; Johnston, Zoe C.; Bellingham, Michelle; Hough, Denise; Walker, Natasha; Filis, Panagiotis; Fowler, Paul A. (2019). Rawlins, Emma (ed.). "Alternative (backdoor) androgen production and masculinization in the human fetus". PLOS Biology. 17 (2): e3000002. doi:10.1371/journal.pbio.3000002. PMC 6375548. PMID 30763313.
- Wilson, Jean D.; Auchus, Richard J.; Leihy, Michael W.; Guryev, Oleg L.; Estabrook, Ronald W.; Osborn, Susan M.; Shaw, Geoffrey; Renfree, Marilyn B. (2003). "5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate". Endocrinology. 144 (2): 575–80. doi:10.1210/en.2002-220721. PMID 12538619. S2CID 84765868.
- Barnard, L.; Gent, R.; Van Rooyen, D.; Swart, A. C. (2017). "Adrenal C11-oxy C(21) steroids contribute to the C11-oxy C(19) steroid pool via the backdoor pathway in the biosynthesis and metabolism of 21-deoxycortisol and 21-deoxycortisone". The Journal of Steroid Biochemistry and Molecular Biology. 174: 86–95. doi:10.1016/j.jsbmb.2017.07.034. PMID 28774496. S2CID 24071400.
- Miller, WL; Auchus, RJ (April 2019). "The "backdoor pathway" of androgen synthesis in human male sexual development". PLOS Biology. 17 (4): e3000198. doi:10.1371/journal.pbio.3000198. PMC 6464227. PMID 30943210.
- Kamrath, Clemens; Hochberg, Ze'ev; Hartmann, Michaela F.; Remer, Thomas; Wudy, Stefan A. (2012). "Increased activation of the alternative "backdoor" pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis". The Journal of Clinical Endocrinology and Metabolism. 97 (3): E367–375. doi:10.1210/jc.2011-1997. ISSN 1945-7197. PMID 22170725. S2CID 3162065.
- Miller, Walter L.; Auchus, Richard J. (2019). "The "backdoor pathway" of androgen synthesis in human male sexual development". PLOS Biology. 17 (4): e3000198. doi:10.1371/journal.pbio.3000198. PMC 6464227. PMID 30943210.
- Shaw, G.; Renfree, M. B.; Leihy, M. W.; Shackleton, C. H.; Roitman, E.; Wilson, J. D. (2000). "Prostate formation in a marsupial is mediated by the testicular androgen 5 alpha-androstane-3 alpha,17 beta-diol". Proceedings of the National Academy of Sciences of the United States of America. 97 (22): 12256–12259. Bibcode:2000PNAS...9712256S. doi:10.1073/pnas.220412297. ISSN 0027-8424. PMC 17328. PMID 11035809.
- Mahendroo, Mala; Wilson, Jean D.; Richardson, James A.; Auchus, Richard J. (2004). "Steroid 5alpha-reductase 1 promotes 5alpha-androstane-3alpha,17beta-diol synthesis in immature mouse testes by two pathways". Molecular and Cellular Endocrinology. 222 (1–2): 113–120. doi:10.1016/j.mce.2004.04.009. ISSN 0303-7207. PMID 15249131. S2CID 54297812.
- Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, Biason-Lauber A (August 2011). "Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation". American Journal of Human Genetics. 89 (2): 201–18. doi:10.1016/j.ajhg.2011.06.009. PMC 3155178. PMID 21802064.
- Reisch, Nicole; Taylor, Angela E.; Nogueira, Edson F.; Asby, Daniel J.; Dhir, Vivek; Berry, Andrew; Krone, Nils; Auchus, Richard J.; Shackleton, Cedric H. L. (2019). "Alternative pathway androgen biosynthesis and human fetal female virilization". Proceedings of the National Academy of Sciences of the United States of America. 116 (44): 22294–22299. Bibcode:2019PNAS..11622294R. doi:10.1073/pnas.1906623116. ISSN 1091-6490. PMC 6825302. PMID 31611378.
- Sumińska, Marta; Bogusz-Górna, Klaudia; Wegner, Dominika; Fichna, Marta (2020). "Non-Classic Disorder of Adrenal Steroidogenesis and Clinical Dilemmas in 21-Hydroxylase Deficiency Combined with Backdoor Androgen Pathway. Mini-Review and Case Report". Int J Mol Sci. 21 (13): 4622. doi:10.3390/ijms21134622. PMC 7369945. PMID 32610579.
- Fukami, Maki; Homma, Keiko; Hasegawa, Tomonobu; Ogata, Tsutomu (2013). "Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development". Developmental Dynamics. 242 (4): 320–9. doi:10.1002/dvdy.23892. PMID 23073980. S2CID 44702659.
- Biswas, Michael G.; Russell, David W. (1997). "Expression cloning and characterization of oxidative 17beta- and 3alpha-hydroxysteroid dehydrogenases from rat and human prostate". J Biol Chem. 272 (25): 15959–66. doi:10.1074/jbc.272.25.15959. PMID 9188497.
- Muthusamy, Selvaraj; Andersson, Stefan; Kim, Hyun-Jin; Butler, Ryan; Waage, Linda; Bergerheim, Ulf; Gustafsson, Jan-Åke (2011). "Estrogen receptor β and 17β-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer". Proc Natl Acad Sci U S A. 108 (50): 20090–4. Bibcode:2011PNAS..10820090M. doi:10.1073/pnas.1117772108. PMC 3250130. PMID 22114194.
- Storbeck, Karl-Heinz; Mostaghel, Elahe A. (2019). "Canonical and Noncanonical Androgen Metabolism and Activity". Advances in Experimental Medicine and Biology. 1210: 239–277. doi:10.1007/978-3-030-32656-2_11. ISBN 978-3-030-32655-5. PMID 31900912. S2CID 209748543.
- Rižner, Tea Lanišnik; Lin, Hsueh K.; Penning, Trevor M. (2003). "Role of human type 3 3alpha-hydroxysteroid dehydrogenase (AKR1C2) in androgen metabolism of prostate cancer cells". Chem Biol Interact. 143–144: 401–9. doi:10.1016/s0009-2797(02)00179-5. PMID 12604227.
- Snaterse, Gido; Mies, Rosinda; van Weerden, Wytske M.; French, Pim J.; Jonker, Johan W.; Houtsmuller, Adriaan B.; van Royen, Martin E.; Visser, Jenny A.; Hofland, Johannes (2022-01-19). "Androgen receptor mutations modulate activation by 11-oxygenated androgens and glucocorticoids". Prostate Cancer and Prostatic Diseases. doi:10.1038/s41391-022-00491-z. ISSN 1476-5608. PMID 35046557. S2CID 246040148.
- Handelsman, David J.; Cooper, Elliot R.; Heather, Alison K. (April 2022). "Bioactivity of 11 keto and hydroxy androgens in yeast and mammalian host cells". The Journal of Steroid Biochemistry and Molecular Biology. 218: 106049. doi:10.1016/j.jsbmb.2021.106049. ISSN 1879-1220. PMID 34990809. S2CID 245635429.
- "Unpublished results communicated by Karl-Heinz Storbeck in review". 21 October 2022.
- Rege, Juilee; Nakamura, Yasuhiro; Satoh, Fumitoshi; Morimoto, Ryo; Kennedy, Michael R.; Layman, Lawrence C.; Honma, Seijiro; Sasano, Hironobu; Rainey, William E. (2013). "Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation". J Clin Endocrinol Metab. 98 (3): 1182–8. doi:10.1210/jc.2012-2912. PMC 3590473. PMID 23386646.
- Campana, Carmela; Rege, Juilee; Turcu, Adina F.; Pezzi, Vincenzo; Gomez-Sanchez, Celso E.; Robins, Diane M.; Rainey, William E. (2016). "Development of a novel cell based androgen screening model". J Steroid Biochem Mol Biol. 156: 17–22. doi:10.1016/j.jsbmb.2015.11.005. PMC 4748855. PMID 26581480.
- Lee, Hyun Gyung; Kim, Chan Jong (2022). "Classic and backdoor pathways of androgen biosynthesis in human sexual development". Ann Pediatr Endocrinol Metab. 27 (2): 83–89. doi:10.6065/apem.2244124.062. PMC 9260366. PMID 35793998. S2CID 250155674.
- Turcu, Adina F.; Auchus, Richard J. (2015). "Adrenal Steroidogenesis and Congenital Adrenal Hyperplasia". Endocrinology and Metabolism Clinics of North America. Elsevier BV. 44 (2): 275–296. doi:10.1016/j.ecl.2015.02.002. ISSN 0889-8529. PMC 4506691. PMID 26038201.
- Turcu, A. F.; Rege, J.; Chomic, R.; Liu, J.; Nishimoto, H. K.; Else, T.; Moraitis, A. G.; Palapattu, G. S.; Rainey, W. E.; Auchus, R. J. (2015). "Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency". The Journal of Clinical Endocrinology and Metabolism. 100 (6): 2283–2290. doi:10.1210/jc.2015-1023. PMC 4454804. PMID 25850025.
- Nguyen, Lee S.; Rouas-Freiss, Nathalie; Funck-Brentano, Christian; Leban, Monique; Carosella, Edgardo D.; Touraine, Philippe; Varnous, Shaida; Bachelot, Anne; Salem, Joe-Elie (2019). "Influence of hormones on the immunotolerogenic molecule HLA-G: a cross-sectional study in patients with congenital adrenal hyperplasia". Eur J Endocrinol. 181 (5): 481–488. doi:10.1530/EJE-19-0379. PMID 31505456. S2CID 202555340.
- Kawarai, Yoshimasa; Ishikawa, Hiroshi; Segawa, Tomoya; Teramoto, Shokichi; Tanaka, Tomoaki; Shozu, Makio (2017). "High serum progesterone associated with infertility in a woman with nonclassic congenital adrenal hyperplasia". J Obstet Gynaecol Res. 43 (5): 946–950. doi:10.1111/jog.13288. PMID 28188961. S2CID 22366495.
- Van Rooyen, Desmaré; Yadav, Rahul; Scott, Emily E.; Swart, Amanda C. (2020). "CYP17A1 exhibits 17αhydroxylase/17,20-lyase activity towards 11β-hydroxyprogesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology. 199: 105614. doi:10.1016/j.jsbmb.2020.105614. PMID 32007561. S2CID 210955834.
- Fiet, J.; Gueux, B.; Raux-DeMay, M. C.; Kuttenn, F.; Vexiau, P.; Brerault, J. L.; Couillin, P.; Galons, H.; Villette, J. M. (March 1989). "Increased plasma 21-deoxycorticosterone (21-DB) levels in late-onset adrenal 21-hydroxylase deficiency suggest a mild defect of the mineralocorticoid pathway". The Journal of Clinical Endocrinology and Metabolism. 68 (3): 542–547. doi:10.1210/jcem-68-3-542. ISSN 0021-972X. PMID 2537337.
- Fiet, J.; Gueux, B.; Raux-Demay, M. C.; Kuttenn, F.; Vexiau, P.; Gourmelen, M.; Couillin, P.; Mornet, E.; Villette, J. M. (1989-12-02). "[21-deoxycortisol. A new marker of virilizing adrenal hyperplasia caused by 21-hydroxylase deficiency]". Presse Medicale (Paris, France: 1983). 18 (40): 1965–1969. ISSN 0755-4982. PMID 2531882.
- Fiet, Jean; Le Bouc, Yves; Guéchot, Jérôme; Hélin, Nicolas; Maubert, Marie-Anne; Farabos, Dominique; Lamazière, Antonin (2017-03-01). "A Liquid Chromatography/Tandem Mass Spectometry Profile of 16 Serum Steroids, Including 21-Deoxycortisol and 21-Deoxycorticosterone, for Management of Congenital Adrenal Hyperplasia". Journal of the Endocrine Society. 1 (3): 186–201. doi:10.1210/js.2016-1048. ISSN 2472-1972. PMC 5686660. PMID 29264476.
- Turcu, Adina F.; Nanba, Aya T.; Chomic, Robert; Upadhyay, Sunil K.; Giordano, Thomas J.; Shields, James J.; Merke, Deborah P.; Rainey, William E.; Auchus, Richard J. (2016). "Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency". Eur J Endocrinol. 174 (5): 601–9. doi:10.1530/EJE-15-1181. PMC 4874183. PMID 26865584.
- Auchus, Richard J.; Buschur, Elizabeth O.; Chang, Alice Y.; Hammer, Gary D.; Ramm, Carole; Madrigal, David; Wang, George; Gonzalez, Martha; Xu, Xu Steven; Smit, Johan W.; Jiao, James; Yu, Margaret K. (2014). "Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency". J Clin Endocrinol Metab. 99 (8): 2763–70. doi:10.1210/jc.2014-1258. PMC 4121028. PMID 24780050.
- Turcu, Adina F.; Mallappa, Ashwini; Nella, Aikaterini A.; Chen, Xuan; Zhao, Lili; Nanba, Aya T.; Byrd, James Brian; Auchus, Richard J.; Merke, Deborah P. (2021). "24-Hour Profiles of 11-Oxygenated C19 Steroids and Δ5-Steroid Sulfates during Oral and Continuous Subcutaneous Glucocorticoids in 21-Hydroxylase Deficiency". Front Endocrinol (Lausanne). 12: 751191. doi:10.3389/fendo.2021.751191. PMC 8636728. PMID 34867794.
- Turcu, Adina F; Mallappa, Ashwini; Elman, Meredith S; Avila, Nilo A; Marko, Jamie; Rao, Hamsini; Tsodikov, Alexander; Auchus, Richard J; Merke, Deborah P (2017). "11-Oxygenated Androgens Are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency". The Journal of Clinical Endocrinology and Metabolism. 102 (8): 2701–2710. doi:10.1210/jc.2016-3989. PMC 5546849. PMID 28472487.
- Schröder, Mariska A M.; Turcu, Adina F.; o'Day, Patrick; Van Herwaarden, Antonius E.; Span, Paul N.; Auchus, Richard J.; Sweep, Fred C G J.; Claahsen-Van Der Grinten, Hedi L. (2022). "Production of 11-Oxygenated Androgens by Testicular Adrenal Rest Tumors". J Clin Endocrinol Metab. 107 (1): e272–e280. doi:10.1210/clinem/dgab598. PMC 8684463. PMID 34390337.
- Flück, Christa E.; Pandey, Amit V. (2014). "Steroidogenesis of the testis -- new genes and pathways". Ann Endocrinol (Paris). 75 (2): 40–7. doi:10.1016/j.ando.2014.03.002. PMID 24793988.
- Zachmann, M. (1996). "Prismatic cases: 17,20-desmolase (17,20-lyase) deficiency". J Clin Endocrinol Metab. 81 (2): 457–9. doi:10.1210/jcem.81.2.8636249. PMID 8636249.
- Boettcher, Claudia; Flück, Christa E. (2022). "Rare forms of genetic steroidogenic defects affecting the gonads and adrenals". Best Pract Res Clin Endocrinol Metab. 36 (1): 101593. doi:10.1016/j.beem.2021.101593. PMID 34711511. S2CID 242536877.
- Du Toit, Therina; Swart, Amanda C. (2021). "Turning the spotlight on the C11-oxy androgens in human fetal development". J Steroid Biochem Mol Biol. 212: 105946. doi:10.1016/j.jsbmb.2021.105946. PMID 34171490. S2CID 235603586.
- Finkielstain, Gabriela P.; Vieites, Ana; Bergadá, Ignacio; Rey, Rodolfo A. (2021). "Disorders of Sex Development of Adrenal Origin". Front Endocrinol (Lausanne). 12: 770782. doi:10.3389/fendo.2021.770782. PMC 8720965. PMID 34987475.
- Eckstein, B.; Borut, A.; Cohen, S. (1987). "Metabolic pathways for androstanediol formation in immature rat testis microsomes". Biochimica et Biophysica Acta (BBA) - General Subjects. 924 (1): 1–6. doi:10.1016/0304-4165(87)90063-8. ISSN 0006-3002. PMID 3828389.
- Penning, Trevor M. (1997). "Molecular Endocrinology of Hydroxysteroid Dehydrogenases". Endocrine Reviews. 18 (3): 281–305. doi:10.1210/edrv.18.3.0302. ISSN 0163-769X. PMID 9183566. S2CID 29607473.
- Barnard, Lise; Gent, Rachelle; Van Rooyen, Desmaré; Swart, Amanda C. (2017). "Adrenal C11-oxy C21 steroids contribute to the C11-oxy C19 steroid pool via the backdoor pathway in the biosynthesis and metabolism of 21-deoxycortisol and 21-deoxycortisone". The Journal of Steroid Biochemistry and Molecular Biology. 174: 86–95. doi:10.1016/j.jsbmb.2017.07.034. PMID 28774496. S2CID 24071400.
- van Rooyen, Desmaré; Gent, Rachelle; Barnard, Lise; Swart, Amanda C. (2018). "The in vitro metabolism of 11β-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology. 178: 203–212. doi:10.1016/j.jsbmb.2017.12.014. PMID 29277707. S2CID 3700135.
