Cyclopentadienyl anion
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of [C
5H
5]−
and abbreviated as Cp−.[1] It is formed by the deprotonation of cyclopentadiene. The cyclopentadienyl anion is a ligand which binds to a metal in organometallic chemistry.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| [C 5H 5]− or Cp− | |||
| Molar mass | 65.09 g/mol | ||
| Conjugate acid | Cyclopentadiene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Resonance and aromaticity

The cyclopentadienyl anion is a planar, cyclic, regular-pentagonal ion; it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. Each double bond and lone pair provides 2 π-electrons, which are delocalizes into the ring.[2] The cyclopentadienyl anion is a conjugated system because there are alternating π and 𝜎 bonds.[3]
Cyclopentadiene has a pKa of about 16. It is acidic relative to many carbon acids. The enhanced acidity is attributed to stabilization of the conjugate base, cyclopentadienyl anion.
Ligand Characteristics
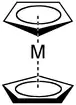
The cyclopentadienyl anion is a common ligand.[4] Cyclopentadienyl complexes are often prepared by salt metathesis reactions using lithium and sodium cyclopentadienide salt. The cyclopentadienyl anion can be viewed as a polydentate ligand when it binds in the [[hapticity|η5] bonding mode.[5][6] The carbon with the lone pair and negative formal charge is an X-type ligand (anionic electron donor) and both double bonds are L-type (neutral electron donors). Since the cyclopentadienyl anion has two L-type ligands and one X-type ligand, it is an L2X ligand.[5][6] The cyclopentadienyl anion has 5 atoms in an uninterrupted π system that bind to a metal, so it has a maximum hapticity of 5.
See also
- Cyclopentadienyl radical, [C
5H
5]• - Cyclopentadienyl cation, [C
5H
5]+ - Cyclooctatetraenide anion, [C
8H
8]2−
References
- "Cyclopentadienide". PubChem Compound Database. National Center for Biotechnology Information. Retrieved 14 April 2016.
- Paul, Satadal; Goswami, Tamal; Misra, Anirban (2015-10-01). "Noncomparative scaling of aromaticity through electron itinerancy". AIP Advances. 5 (10): 107211. doi:10.1063/1.4933191.
- "Delocalised electrons- Definition and Examples of Delocalized electrons with FAQs". BYJUS. Retrieved 2023-04-01.
- "Cyclopentadienyl Anion - Introduction, Cyclopentadienyl Anion Complexes, Synthesis and Applications of Cyclopentadienyl Anion". BYJUS. Retrieved 2023-04-01.
- "13.3.2: Simplifying the Organometallic Complex by Deconstruction". Chemistry LibreTexts. 2022-06-14. Retrieved 2023-04-01.
- "Simplifying the Organometallic Complex (Part 3)". Chemistry LibreTexts. 2013-10-02. Retrieved 2023-04-14.

