Matter wave
Matter waves are a central part of the theory of quantum mechanics, being an example of wave–particle duality. All matter exhibits wave-like behavior. For example, a beam of electrons can be diffracted just like a beam of light or a water wave.
| Part of a series of articles about |
| Quantum mechanics |
|---|
The concept that matter behaves like a wave was proposed by French physicist Louis de Broglie (/dəˈbrɔɪ/) in 1924, and so matter waves are also known as de Broglie waves.
The de Broglie wavelength is the wavelength, λ, associated with a particle with momentum p through the Planck constant, h:
Wave-like behavior of matter was first experimentally demonstrated by George Paget Thomson and Alexander Reid's transmission diffraction experiment,[1] and independently in the Davisson–Germer experiment,[2][3] both using electrons; and it has also been confirmed for other elementary particles, neutral atoms and molecules.
History
Background
At the end of the 19th century, light was thought to consist of waves of electromagnetic fields which propagated according to Maxwell's equations, while matter was thought to consist of localized particles (see history of wave and particle duality). In 1900, this division was questioned when, investigating the theory of black-body radiation, Max Planck proposed that the thermal energy of oscillating atoms is divided into discrete portions, or quanta.[4] Extending Planck's investigation in several ways, including its connection with the photoelectric effect, Albert Einstein proposed in 1905 that light is also propagated and absorbed in quanta, now called photons. These quanta would have an energy given by the Planck–Einstein relation:
and a momentum
where ν (lowercase Greek letter nu) and λ (lowercase Greek letter lambda) denote the frequency and wavelength of the light, c the speed of light, and h the Planck constant.[5] In the modern convention, frequency is symbolized by f as is done in the rest of this article. Einstein's postulate was confirmed experimentally by Robert Millikan and Arthur Compton over the next two decades.
De Broglie hypothesis

De Broglie, in his 1924 PhD thesis,[6] proposed that just as light has both wave-like and particle-like properties, electrons also have wave-like properties. He took the result from special relativity that a body of rest mass m0 contains an energy E = m0c2 in its rest frame and combined this with the idea that the energy of a quantum is proportional to its frequency. So, he posited, each body rest mass m0 is associated with some kind of periodic oscillation whose frequency ν0 is set by hν0 = m0c2 in the rest frame.[7][8] Thus, it was a simple step to get to the equation that bears his name.[9] Also, by rearranging the momentum equation stated in the above section, we find a relationship between the wavelength, λ, associated with an electron and its momentum, p, through the Planck constant, h:[10]
The relationship has since been shown to hold for all types of matter: all matter exhibits properties of both particles and waves.
When I conceived the first basic ideas of wave mechanics in 1923–1924, I was guided by the aim to perform a real physical synthesis, valid for all particles, of the coexistence of the wave and of the corpuscular aspects that Einstein had introduced for photons in his theory of light quanta in 1905.
— de Broglie[11]
Following up on de Broglie's ideas, physicist Peter Debye made an offhand comment that if particles behaved as waves, they should satisfy some sort of wave equation. Inspired by Debye's remark, Erwin Schrödinger decided to find a proper three-dimensional wave equation for the electron. He was guided by William Rowan Hamilton's analogy between mechanics and optics (see Hamilton–Jacobi equation), encoded in the observation that the zero-wavelength limit of optics resembles a mechanical system – the trajectories of light rays become sharp tracks that obey Fermat's principle, an analog of the principle of least action.[12] In 1926, Schrödinger published the wave equation that now bears his name[13] – the matter wave analogue of Maxwell's equations – and used it to derive the energy spectrum of hydrogen. Frequencies of solutions of the non-relativistic Schrödinger equation differ from de Broglie waves by the Compton frequency since the energy corresponding to the rest mass of a particle is not part of the non-relativistic Schrödinger equation. The Schrödinger equation describes the time evolution of a wavefunction, a function that assigns a complex number to each point in space. Schrödinger tried to interpret the modulus squared of the wavefunction as a charge density. This approach was, however, unsuccessful.[14][15][16] Max Born proposed that the modulus squared of the wavefunction is instead a probability density, a successful proposal now known as the Born rule.[14]
Experimental confirmation
Matter waves were first experimentally confirmed to occur in George Paget Thomson and Alexander Reid's diffraction experiment[1] and the Davisson–Germer experiment,[2][3] both for electrons. The de Broglie hypothesis and the existence of matter waves has been confirmed for other elementary particles, neutral atoms and even molecules have been shown to be wave-like.
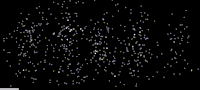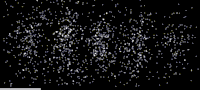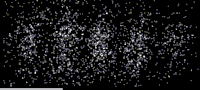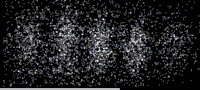
As shown in this image, an especially clear confirmation experiment by Bach et al[17] implemented the famous double-slit experiment[18] using electrons through physical apertures and recorded each particle building up the full diffraction pattern.
Electrons
In 1927 at Bell Labs, Clinton Davisson and Lester Germer fired slow-moving electrons at a crystalline nickel target.[2][3] The angular dependence of the diffracted electron intensity was measured, and was determined to have a similar angular dependence to diffraction patterns predicted by Bragg for x-rays. At the same time George Paget Thomson and Alexander Reid at the University of Aberdeen were independently firing electrons at thin celluloid foils and later metal films, observing rings which can be similarly interpreted.[1] (Alexander Reid, who was Thomson's graduate student, performed the first experiments but he died soon after in a motorcycle accident[19] and is rarely mentioned.) Before the acceptance of the de Broglie hypothesis, diffraction was a property that was thought to be exhibited only by waves. Therefore, the presence of any diffraction effects by matter demonstrated the wave-like nature of matter. When the de Broglie wavelength was inserted into the Bragg condition, a similar angular dependence to the experimentally observed diffraction pattern was found, thereby experimentally supporting the de Broglie hypothesis for electrons.[20] This was placed onto a solid foundation in 1928 by Hans Bethe,[21] who solved the Schrödinger equation,[13] showing how this could explain the experimental results. His approach is similar to what is used in modern electron diffraction approaches.[22][23]
This was a pivotal result in the development of quantum mechanics. Just as the photoelectric effect demonstrated the particle nature of light, the Davisson–Germer experiment showed the wave-nature of matter, and completed the theory of wave–particle duality. This idea was important because it meant that not only could any particle exhibit wave characteristics, but that one could use wave equations to describe phenomena in matter.
Neutrons
Neutrons, produced in nuclear reactors with around 1MeV energy, thermalize to around 0.025eV as they scatter from light atoms. The resulting de Broglie wavelength (around 180 pm) matches interatomic spacing. In 1944, Ernest O. Wollan, with a background in X-ray scattering from his PhD work[24] under Arthur Compton, recognized the potential for applying thermal neutrons from the newly operational X-10 nuclear reactor to crystallography. Joined by Clifford G. Shull they developed[25] neutron diffraction through out the 1940s.
Neutral atoms
Experiments with Fresnel diffraction[26] and an atomic mirror for specular reflection[27][28] of neutral atoms confirm the application of the de Broglie hypothesis to atoms, i.e. the existence of atomic waves which undergo diffraction, interference and allow quantum reflection by the tails of the attractive potential.[29] Advances in laser cooling have allowed cooling of neutral atoms down to nanokelvin temperatures. At these temperatures, the thermal de Broglie wavelengths come into the micrometre range. Using Bragg diffraction of atoms and a Ramsey interferometry technique, the de Broglie wavelength of cold sodium atoms was explicitly measured and found to be consistent with the temperature measured by a different method.[30]
This effect has been used to demonstrate atomic holography, and it may allow the construction of an atom probe imaging system with nanometer resolution.[31][32] The description of these phenomena is based on the wave properties of neutral atoms, confirming the de Broglie hypothesis.
The effect has also been used to explain the spatial version of the quantum Zeno effect, in which an otherwise unstable object may be stabilised by rapidly repeated observations.[28]
Molecules
Recent experiments even confirm the relations for molecules and even macromolecules that otherwise might be supposed too large to undergo quantum mechanical effects. In 1999, a research team in Vienna demonstrated diffraction for molecules as large as fullerenes.[33] The researchers calculated a de Broglie wavelength of the most probable C60 velocity as 2.5 pm. More recent experiments prove the quantum nature of molecules made of 810 atoms and with a mass of 10123 Da.[34] As of 2019, this has been pushed to molecules of 25000 Da.[35]
De Broglie relations
The de Broglie equations relate the wavelength λ to the momentum p, and frequency f to the total energy E of a free particle:[36]
where h is the Planck constant. The equations can also be written as
although it is argued that these would more appropriately be written[37]
where ħ = h/2π is the reduced Planck constant, k is the angular wave vector, β is the phase constant, and ω is the angular frequency.
In each pair, the second equation is also referred to as the Planck–Einstein relation, since it was also proposed by Planck and Einstein.
Special relativity
Using two formulas from special relativity, one for the relativistic mass energy and one for the relativistic momentum
allows the equations to be written as
where denotes the particle's rest mass, its velocity, the Lorentz factor, and the speed of light in vacuum.[38][39][40] See below for details of the derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity (equal to the product of the particle's frequency and its wavelength). In the case of a non-dispersive medium, they happen to be equal, but otherwise they are not.
Group velocity
Albert Einstein first explained the wave–particle duality of light in 1905. Louis de Broglie hypothesized that any particle should also exhibit such a duality. The velocity of a particle, he concluded, should always equal the group velocity of the corresponding wave.
Both in relativistic and non-relativistic quantum physics, we can identify the group velocity of a particle's wave function with the particle velocity. Quantum mechanics has very accurately demonstrated this hypothesis, and the relation has been shown explicitly for particles as large as molecules.[33]
De Broglie deduced that if the duality equations already known for light were the same for any particle, then his hypothesis would hold. This means that
where E is the total energy of the particle, p is its momentum, ħ is the reduced Planck constant. For a free non-relativistic particle it follows that
where m is the mass of the particle and v its velocity.
Also in special relativity we find that
where m0 is the rest mass of the particle and c is the speed of light in vacuum. But (see below), using that the phase velocity is vp = E/p = c2/v, therefore
where v is the velocity of the particle regardless of wave behavior.
Phase velocity
In quantum mechanics, particles also behave as waves with complex amplitudes. The phase velocity is equal to the product of the frequency multiplied by the wavelength.
By the de Broglie hypothesis, we see that
Using relativistic relations for energy and momentum, we have
where E is the total energy of the particle, p the momentum, the Lorentz factor, c the speed of light, and β the speed as a fraction of c. The variable v can either be taken to be the speed of the particle or the group velocity of the corresponding matter wave. Since the particle speed for any particle that has nonzero mass (according to special relativity), the phase velocity of matter waves always exceeds c, i.e.,
which approaches c when the particle speed is relativistic. The superluminal phase velocity does not violate special relativity. See the article on Dispersion (optics) for details.
Four-vectors
Using four-vectors, the De Broglie relations form a single equation:
which is frame-independent.
Likewise, the relation between group/particle velocity and phase velocity is given in frame-independent form by:
where
De Broglie's phase wave and periodic phenomenon
De Broglie's thesis started from the hypothesis, "that to each portion of energy with a proper mass m0 one may associate a periodic phenomenon of the frequency ν0, such that one finds: hν0 = m0c2. The frequency ν0 is to be measured, of course, in the rest frame of the energy packet. This hypothesis is the basis of our theory."[41][42][43][44][45][46] (This frequency is also known as Compton frequency.)
De Broglie followed his initial hypothesis of a periodic phenomenon, with frequency ν0, associated with the energy packet. He used the special theory of relativity to find, in the frame of the observer of the electron energy packet that is moving with velocity , that its frequency was apparently reduced to
De Broglie reasoned that to a stationary observer this hypothetical intrinsic particle periodic phenomenon appears to be in phase with a wave of wavelength and frequency that is propagating with phase velocity . De Broglie called this wave the "phase wave" («onde de phase» in French). This was his basic matter wave conception. He noted, as above, that , and the phase wave does not transfer energy.[43][47]
While the concept of waves being associated with matter is correct, de Broglie did not leap directly to the final understanding of quantum mechanics with no missteps. There are conceptual problems with the approach that de Broglie took in his thesis that he was not able to resolve, despite trying a number of different fundamental hypotheses in different papers published while working on, and shortly after publishing, his thesis.[44][48] These difficulties were resolved by Erwin Schrödinger, who developed the wave mechanics approach, starting from a somewhat different basic hypothesis.
See also
References
- Thomson, G. P.; Reid, A. (1927). "Diffraction of Cathode Rays by a Thin Film". Nature. 119 (3007): 890. Bibcode:1927Natur.119Q.890T. doi:10.1038/119890a0. ISSN 0028-0836. S2CID 4122313.
- Davisson, C.; Germer, L. H. (1927). "Diffraction of Electrons by a Crystal of Nickel". Physical Review. 30 (6): 705–740. Bibcode:1927PhRv...30..705D. doi:10.1103/physrev.30.705. ISSN 0031-899X.
- Davisson, C. J.; Germer, L. H. (1928). "Reflection of Electrons by a Crystal of Nickel". Proceedings of the National Academy of Sciences. 14 (4): 317–322. Bibcode:1928PNAS...14..317D. doi:10.1073/pnas.14.4.317. ISSN 0027-8424. PMC 1085484. PMID 16587341.
- Kragh, Helge (1 December 2000). "Max Planck: the reluctant revolutionary". Physics World. Retrieved 19 May 2023.
- Einstein, A. (1917). Zur Quantentheorie der Strahlung, Physicalische Zeitschrift 18: 121–128. Translated in ter Haar, D. (1967). The Old Quantum Theory. Pergamon Press. pp. 167–183. LCCN 66029628.
- de Broglie, Louis Victor. "On the Theory of Quanta". Foundation of Louis de Broglie (English translation by A.F. Kracklauer, 2004. ed.). Retrieved 25 February 2023.
- de Broglie, L. (1923). "Waves and quanta". Nature. 112 (2815): 540. Bibcode:1923Natur.112..540D. doi:10.1038/112540a0. S2CID 4082518.
- de Broglie, Louis (1925). "Recherches sur la théorie des Quanta". Annales de Physique (in French). 10 (3): 33. Bibcode:1925AnPh...10...22D. doi:10.1051/anphys/192510030022. ISSN 0003-4169., translated in 2004 by A. F. Kracklauer as De Broglie, Louis, On the Theory of Quanta, p. 8
- R. Nave, “Wave Nature of Electron, Hyperphysics.com http://hyperphysics.phy-astr.gsu.edu/hbase/debrog.html#c3
- McEvoy, J. P.; Zarate, Oscar (2004). Introducing Quantum Theory. Totem Books. pp. 110–114. ISBN 978-1-84046-577-8.
- de Broglie, Louis (1970). "The reinterpretation of wave mechanics". Foundations of Physics. 1 (1): 5–15. Bibcode:1970FoPh....1....5D. doi:10.1007/BF00708650. S2CID 122931010.
- Schrödinger, E. (1984). Collected papers. Friedrich Vieweg und Sohn. ISBN 978-3-7001-0573-2. See the introduction to first 1926 paper.
- Schrödinger, E. (1926). "An Undulatory Theory of the Mechanics of Atoms and Molecules". Physical Review. 28 (6): 1049–1070. Bibcode:1926PhRv...28.1049S. doi:10.1103/PhysRev.28.1049. ISSN 0031-899X.
- Moore, W. J. (1992). Schrödinger: Life and Thought. Cambridge University Press. pp. 219–220. ISBN 978-0-521-43767-7.
- Jammer, Max (1974). Philosophy of Quantum Mechanics: The interpretations of quantum mechanics in historical perspective. Wiley-Interscience. pp. 24–25. ISBN 9780471439585.
- Karam, Ricardo (June 2020). "Schrödinger's original struggles with a complex wave function". American Journal of Physics. 88 (6): 433–438. doi:10.1119/10.0000852. ISSN 0002-9505. S2CID 219513834.
- Bach, Roger; Pope, Damian; Liou, Sy-Hwang; Batelaan, Herman (13 March 2013). "Controlled double-slit electron diffraction". New Journal of Physics. IOP Publishing. 15 (3): 033018. doi:10.1088/1367-2630/15/3/033018. ISSN 1367-2630.
- Born, M.; Wolf, E. (1999). Principles of Optics. Cambridge University Press. ISBN 978-0-521-64222-4.
- Navarro, Jaume (2010). "Electron diffraction chez Thomson: early responses to quantum physics in Britain". The British Journal for the History of Science. 43 (2): 245–275. doi:10.1017/S0007087410000026. ISSN 0007-0874.
- Mauro Dardo, Nobel Laureates and Twentieth-Century Physics, Cambridge University Press 2004, pp. 156–157
- Bethe, H. (1928). "Theorie der Beugung von Elektronen an Kristallen". Annalen der Physik (in German). 392 (17): 55–129. Bibcode:1928AnP...392...55B. doi:10.1002/andp.19283921704.
- John M., Cowley (1995). Diffraction physics. Elsevier. ISBN 0-444-82218-6. OCLC 247191522.
- Peng, L.-M.; Dudarev, S. L.; Whelan, M. J. (2011). High energy electron diffraction and microscopy. Oxford: Oxford University Press. ISBN 978-0-19-960224-7. OCLC 656767858.
- Snell, A. H.; Wilkinson, M. K.; Koehler, W. C. (1984). "Ernest Omar Wollan". Physics Today. 37 (11): 120. Bibcode:1984PhT....37k.120S. doi:10.1063/1.2915947.
- Shull, C. G. (1997). "Early Development of Neutron Scattering" (PDF). In Ekspong, G. (ed.). Nobel Lectures, Physics 1991–1995. World Scientific Publishing. pp. 145–154. Archived from the original (PDF) on 19 May 2017.
- R.B.Doak; R.E.Grisenti; S.Rehbein; G.Schmahl; J.P.Toennies; Ch. Wöll (1999). "Towards Realization of an Atomic de Broglie Microscope: Helium Atom Focusing Using Fresnel Zone Plates". Physical Review Letters. 83 (21): 4229–4232. Bibcode:1999PhRvL..83.4229D. doi:10.1103/PhysRevLett.83.4229.
- F. Shimizu (2000). "Specular Reflection of Very Slow Metastable Neon Atoms from a Solid Surface". Physical Review Letters. 86 (6): 987–990. Bibcode:2001PhRvL..86..987S. doi:10.1103/PhysRevLett.86.987. PMID 11177991. S2CID 34195829.
- D. Kouznetsov; H. Oberst (2005). "Reflection of Waves from a Ridged Surface and the Zeno Effect". Optical Review. 12 (5): 1605–1623. Bibcode:2005OptRv..12..363K. doi:10.1007/s10043-005-0363-9. S2CID 55565166.
- H.Friedrich; G.Jacoby; C.G.Meister (2002). "quantum reflection by Casimir–Van der Waals potential tails". Physical Review A. 65 (3): 032902. Bibcode:2002PhRvA..65c2902F. doi:10.1103/PhysRevA.65.032902.
- Pierre Cladé; Changhyun Ryu; Anand Ramanathan; Kristian Helmerson; William D. Phillips (2008). "Observation of a 2D Bose Gas: From thermal to quasi-condensate to superfluid". Physical Review Letters. 102 (17): 170401. arXiv:0805.3519. Bibcode:2009PhRvL.102q0401C. doi:10.1103/PhysRevLett.102.170401. PMID 19518764. S2CID 19465661.
- Shimizu; J.Fujita (2002). "Reflection-Type Hologram for Atoms". Physical Review Letters. 88 (12): 123201. Bibcode:2002PhRvL..88l3201S. doi:10.1103/PhysRevLett.88.123201. PMID 11909457.
- D. Kouznetsov; H. Oberst; K. Shimizu; A. Neumann; Y. Kuznetsova; J.-F. Bisson; K. Ueda; S. R. J. Brueck (2006). "Ridged atomic mirrors and atomic nanoscope". Journal of Physics B. 39 (7): 1605–1623. Bibcode:2006JPhB...39.1605K. CiteSeerX 10.1.1.172.7872. doi:10.1088/0953-4075/39/7/005.
- Arndt, M.; O. Nairz; J. Voss-Andreae; C. Keller; G. van der Zouw; A. Zeilinger (14 October 1999). "Wave–particle duality of C60". Nature. 401 (6754): 680–682. Bibcode:1999Natur.401..680A. doi:10.1038/44348. PMID 18494170. S2CID 4424892.
- Eibenberger, Sandra; Gerlich, Stefan; Arndt, Markus; Mayor, Marcel; Tüxen, Jens (14 August 2013). "Matter–wave interference of particles selected from a molecular library with masses exceeding 10000 amu". Physical Chemistry Chemical Physics. 15 (35): 14696–700. arXiv:1310.8343. Bibcode:2013PCCP...1514696E. doi:10.1039/c3cp51500a. ISSN 1463-9084. PMID 23900710. S2CID 3944699.
- "2000 atoms in two places at once: A new record in quantum superposition". phys.org. Retrieved 25 September 2019.
- Resnick, R.; Eisberg, R. (1985). Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles (2nd ed.). New York: John Wiley & Sons. ISBN 978-0-471-87373-0.
- Z.Y.Wang (2016). "Generalized momentum equation of quantum mechanics". Optical and Quantum Electronics. 48 (2). doi:10.1007/s11082-015-0261-8. S2CID 124732329.
- Holden, Alan (1971). Stationary states. New York: Oxford University Press. ISBN 978-0-19-501497-6.
- Williams, W.S.C. (2002). Introducing Special Relativity, Taylor & Francis, London, ISBN 0-415-27761-2, p. 192.
- De Broglie, Louis (1970). "The reinterpretation of wave mechanics". Foundations of Physics. 1 (1): 5–15. Bibcode:1970FoPh....1....5D. doi:10.1007/BF00708650. S2CID 122931010.
- de Broglie, L. (1923). "Waves and quanta". Nature. 112 (2815): 540. Bibcode:1923Natur.112..540D. doi:10.1038/112540a0. S2CID 4082518.
- de Broglie, L. (1924). Thesis, p. 8 of Kracklauer's translation.
- Medicus, H.A. (1974). "Fifty years of matter waves". Physics Today. 27 (2): 38–45. Bibcode:1974PhT....27b..38M. doi:10.1063/1.3128444.
- MacKinnon, E. (1976). De Broglie's thesis: a critical retrospective, Am. J. Phys. 44: 1047–1055.
- Espinosa, J.M. (1982). "Physical properties of de Broglie's phase waves". Am. J. Phys. 50 (4): 357–362. Bibcode:1982AmJPh..50..357E. doi:10.1119/1.12844.
- Brown, H.R.; Martins (1984). "De Broglie's relativistic phase waves and wave groups". Am. J. Phys. 52 (12): 1130–1140. Bibcode:1984AmJPh..52.1130B. doi:10.1119/1.13743. Archived from the original on 29 July 2020. Retrieved 16 December 2019.
- Bacciagaluppi, G., Valentini, A. (2009). Quantum Theory at the Crossroads: Reconsidering the 1927 Solvay Conference, Cambridge University Press, Cambridge UK, ISBN 978-0-521-81421-8, pp. 30–88.
- Martins, Roberto de Andrade (2010). "Louis de Broglie's Struggle with the Wave–Particle Dualism, 1923–1925". Quantum History Project, Fritz Haber Institute of the Max Planck Society and the Max Planck Institute for the History of Science. Retrieved 3 January 2015.
Further reading
- L. de Broglie, Recherches sur la théorie des quanta (Researches on the quantum theory), Thesis (Paris), 1924; L. de Broglie, Ann. Phys. (Paris) 3, 22 (1925). English translation by A.F. Kracklauer.
- Broglie, Louis de, The wave nature of the electron Nobel Lecture, 12, 1929
- Tipler, Paul A. and Ralph A. Llewellyn (2003). Modern Physics. 4th ed. New York; W. H. Freeman and Co. ISBN 0-7167-4345-0. pp. 203–4, 222–3, 236.
- Zumdahl, Steven S. (2005). Chemical Principles (5th ed.). Boston: Houghton Mifflin. ISBN 978-0-618-37206-5.
- An extensive review article "Optics and interferometry with atoms and molecules" appeared in July 2009: https://web.archive.org/web/20110719220930/http://www.atomwave.org/rmparticle/RMPLAO.pdf.
- "Scientific Papers Presented to Max Born on his retirement from the Tait Chair of Natural Philosophy in the University of Edinburgh", 1953 (Oliver and Boyd)
External links
- Bowley, Roger. "de Broglie Waves". Sixty Symbols. Brady Haran for the University of Nottingham.