Pentane
Pentane is an organic compound with the chemical formula of C
5H
12. It is an alkane with five carbon atoms. Usually, "pentane" represents all three isomers (n-pentane, isopentane and neopentane). But in the IUPAC, pentane only represents n-pentane. In the IUPAC, the other two isomers is 2-methylbutane and 2,2-dimethylpropane. Cyclopentane's chemical formula is C
5H
10, so it is not an isomer of pentane.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pentane | |||
| Other names
Quintane[1] | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| Beilstein Reference | 969132 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.358 | ||
| EC Number |
| ||
| Gmelin Reference | 1766 | ||
| MeSH | pentane | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1265 | ||
CompTox Dashboard (EPA) |
|||
SMILES
| |||
| Properties[2] | |||
| C5H12 | |||
| Molar mass | 72.15 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Gasoline-like[3] | ||
| Density | 0.626 g mL−1; 0.6262 g mL−1 (at 20 °C) | ||
| Melting point | −130.5 to −129.1 °C; −202.8 to −200.3 °F; 142.7 to 144.1 K | ||
| Boiling point | 35.9 to 36.3 °C; 96.5 to 97.3 °F; 309.0 to 309.4 K | ||
| 40 mg L−1 (at 20 °C) | |||
| log P | 3.255 | ||
| Vapor pressure | 57.90 kPa (at 20.0 °C) | ||
| kH | 7.8 nmol Pa−1 kg−1 | ||
| Acidity (pKa) | ~45 | ||
| Basicity (pKb) | ~59 | ||
| λmax | 200 nm | ||
| -63.05·10−6 cm3/mol | |||
Refractive index (nD) |
1.358 | ||
| Viscosity | 0.240 mPa·s (at 20 °C) | ||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−174.1–−172.9 kJ mol−1 | ||
| Std enthalpy of combustion ΔcH |
−3.5095–−3.5085 MJ mol−1 | ||
| Standard molar entropy S |
263.47 J K−1 mol−1 | ||
| Specific heat capacity, C | 167.19 J K−1 mol−1 | ||
| Hazards | |||
| NFPA 704 |
4
1
0
| ||
| Explosive limits | 1.5–7.8%[3] | ||
| U.S. Permissible exposure limit (PEL) |
TWA 1000 ppm (2950 mg/m3)[3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Isomers
| Common name | normal pentane unbranched pentane n-pentane |
isopentane | neopentane |
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Molecular diagram |
 |
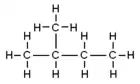 |
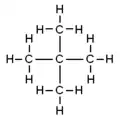 |
| Skeletal diagram |
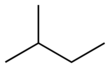 |
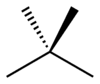 |
Reaction
All isomers of pentane burn with oxygen to make carbon dioxide and water:
- C
5H
12+8O
2->5CO
2+6H
2O.
References
- Hofmann, August Wilhelm Von (1 January 1867). "I. On the action of trichloride of phosphorus on the salts of the aromatic monamines". Proceedings of the Royal Society of London. 15: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840. Retrieved 4 April 2018 – via rspl.royalsocietypublishing.org.
- Record of n-Pentane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 19 April 2011.
- NIOSH Pocket Guide to Chemical Hazards. "#0486". National Institute for Occupational Safety and Health (NIOSH).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.


