2-Ethylhexanoic acid
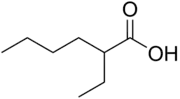
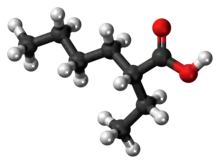
2-Ethylhexanoic acid is the organic compound with the formula CH3(CH2)3CH(C2H5)CO2H. It is a carboxylic acid that is widely used to prepare lipophilic metal derivatives that are soluble in nonpolar organic solvents. 2-Ethylhexanoic acid is a colorless viscous oil. It is supplied as a racemic mixture.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethylhexanoic acid[1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 1750468 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.222 |
| EC Number |
|
| MeSH | 2-ethylhexanoic+acid |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H16O2 | |
| Molar mass | 144.214 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 903 mg mL−1 |
| Melting point | −59.00 °C; −74.20 °F; 214.15 K |
| Boiling point | 228.1 °C; 442.5 °F; 501.2 K |
| log P | 2.579 |
| Vapor pressure | <1 Pa (at 25 °C) |
| Acidity (pKa) | 4.819 |
| Basicity (pKb) | 9.178 |
Refractive index (nD) |
1.425 |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−635.1 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
-4.8013–4.7979 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H312, H318, H361 | |
| P280, P305+P351+P338 | |
| Flash point | 114 °C (237 °F; 387 K) |
| 371 °C (700 °F; 644 K) | |
| Explosive limits | 0.9–6.7% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
2-Ethylhexanoic acid is produced industrially from propylene, which is hydroformylated to give butyraldehyde. Aldol condensation of the aldehyde gives 2-ethylhexenal, which is hydrogenated to 2-ethylhexanal. Oxidation of this aldehyde gives the carboxylic acid.[2]
Metal ethylhexanoates
2.jpg.webp)
2-Ethylhexanoic acid forms compounds with metal cations that have stoichiometry as metal acetates. These ethylhexanoate complexes are used in organic and industrial chemical synthesis.[3] They function as catalysts in polymerizations as well as for oxidation reactions as "oil drying agents."[4] They are highly soluble in nonpolar solvents. These metal complexes are often described as salts. They are, however, not ionic but charge-neutral coordination complexes. Their structures are akin to the corresponding acetates.
Examples of metal ethylhexanoates
- Hydroxyl aluminium bis(2-ethylhexanoate), used as a thickener
- Tin(II) ethylhexanoate (CAS# 301-10-0), a catalyst for polylactide and poly(lactic-co-glycolic acid).[5]
- Cobalt(II) ethylhexanoate (CAS# 136-52-7), a drier for alkyd resins
- Nickel(II) ethylhexanoate (CAS# 4454-16-4)
See also
References
- "2-ethylhexanoic acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 21 February 2012.
- Riemenschneider, Wilhelm (2002). "Carboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_235. ISBN 978-3527306732.
- Mishra, Shashank; Daniele, Stéphane; Hubert-Pfalzgraf, Liliane G. (2007). "Metal 2-Ethylhexanoates and Related Compounds as Useful Precursors in Materials Science". Chemical Society Reviews. 36 (11): 1770–1787. doi:10.1039/B614334M. PMID 18213985.
- Raju, Ravinder; Prasad, Kapa (2012). "Synthetic applications of 2-ethylhexanoic acid derived reagents". Tetrahedron. 68 (5): 1341–1349. doi:10.1016/j.tet.2011.10.078.
- Coulembier, O.; Degee, P.; Hedrick, J. L.; Dubois, P. (2006). "Controlled Ring-Opening Polymerization to Biodegradable Aliphatic Polyester: Especially Poly(Β-Malic Acid) Derivatives". Prog. Polym. Sci. 31: 723–747. doi:10.1016/j.progpolymsci.2006.08.004.