wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time.
This article has been viewed 31,844 times.
Learn more...
Mass defect (also referred to as mass deficit) is a phenomenon which occurs in physics. When we calculate the theoretical mass of a nucleus using the masses of protons and neutrons and compare it to the experimental mass, we observe that there is a small, but relevant, difference in the two masses. This is a result from the binding energy which is responsible for binding protons and neutrons together in the nucleus of an atom. Simply put, some of the mass from the protons and neutrons in the nucleus is converted to the binding energy (according to Einstein's E=mc^2).[1]
Steps
Calculate the Theoretical Mass
-
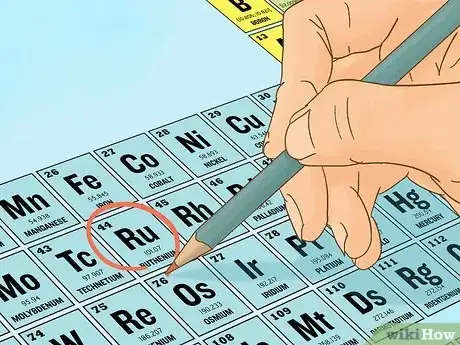
1Determine how many protons and neutrons are found in the isotope in question. Use a periodic table and remember that certain isotopes of an element may have a different number of neutrons.
-
2Calculate the total theoretical mass of the nucleus. Multiply the amount of protons by its mass in atomic mass units (approximately 1.007276u) and add it to the amount of neutrons multiplied by its mass (approximately 1.008665u). Note that your teacher may provide you a specific value to use for these masses, or specify how many significant digits you are required to use.[2]
Community Q&A
-
QuestionHow do I calculate binding energy?
 Community AnswerFirst, calculate the mass defect. Then, multiply the answer by 931 MeV/u. This is the amount of energy with 1 u mass. The answer you get will be the binding energy.
Community AnswerFirst, calculate the mass defect. Then, multiply the answer by 931 MeV/u. This is the amount of energy with 1 u mass. The answer you get will be the binding energy.