wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time.
This article has been viewed 3,560 times.
Learn more...
This guide will provide an introduction to definitions, basic amino acids and an example of how to draw a dipeptide. A dipeptide consists of two amino acid residues while polypeptides consist of more than two. These structures are important because they help make up proteins in the body and also have roles in reaction mechanisms. By drawing the backbone and functional groups, detailed steps will be given to learn how to alter those groups based on a specific pH, a scale to measure acidity or basicity of a solution. By knowing how the structure will look like under certain conditions, this will give students a better understanding of how they are used in reactions. This wikiHow will also help in drawing peptides consisting of more than two amino acids.
Steps
Learning definitions and the basic structures.
-
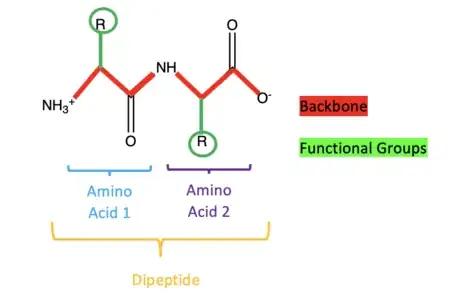
1Know the basic definitions. A dipeptide is an organic compound consisting of two amino acids. It has two components, a backbone and attached functional groups.
-
2Learn the 20 basic amino acids. The 20 amino acids can be found through an internet search. Learn their structures, their three-letter abbreviations and their functional groups which will later be used to replace the "R" in the basic structure.
Drawing the Basic Dipeptide
-
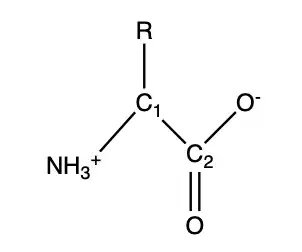
1Draw a single amino acid. Each amino starts with an amine end (NH3+) and a carbonyl end (COO-). Starting with the amine end, write "NH3+" and from there, draw a line up to the right, creating a point called C1 to represent carbon 1. Then from C1, draw a line down and to the right to create another point called C2. Lastly, from C2 draw a line up to the right and at the end of the line write "O-". At C1 draw a line straight up and write "R" to represent a functional group that will later be replaced. At C2, draw two lines straight down and write "O". This completes a drawing of a single amino acid.
-
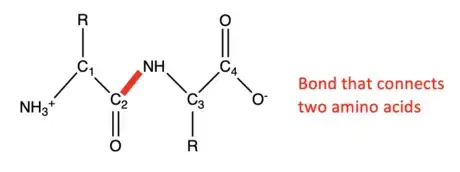
2Connect two amino acids. To create a dipeptide, the single amino acids must be connected. Start the drawing again as stated in the previous step, but at C2, draw the line up to the right and write "NH" at the end instead of "O". From there, draw a line down and to the right to create a point called C3. At C3, a line will be drawn up to the right to create a point called C4. Lastly, a line will be drawn down to the right and at the end of the line write "O-". At C3, draw a line straight down and write "R" , which will later be replaced by a functional group. At C4, draw two lines straight up and write "O" at the end. This completes a drawing of a basic dipeptide.
Adding the Functional Groups
-
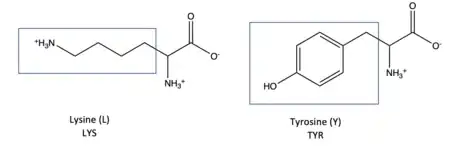
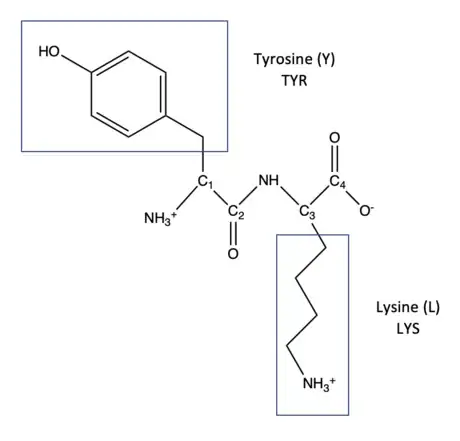
1Look at the three-letter abbreviation and choose the correct amino acid. For example, the dipeptide Tyr-Lys will be used as an example. The correct amino acids are drawn below.
-
2Replace "R" with the amino acid functional groups. At C1, the functional group of Tyr will be drawn to replace "R". At C3, the functional group of Lys will be drawn to replace "R".
-
3Know how pH alters ends and functional groups. Based on the pH a dipeptide is at, and the relative pKa values, this determines if the amine end, carbonyl end and specific functional groups are protonated (H added) or deprotonated (H taken away).
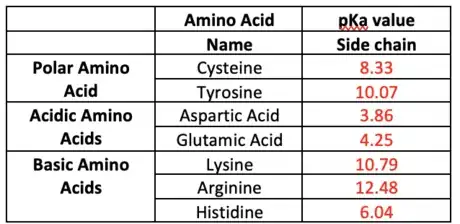
If pH< pKa, then it is protonated. If pH>pKa, then it is deprotonated. A table of the amino acid pKa values are shown but the focus will be on the pKa of the side chains indicated in red.
-
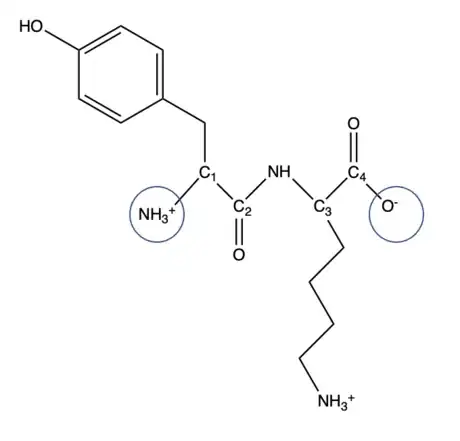
1Alter ends based on specific pH. The pH in this example will be pH 7. For the amine end, the pKa will always be 9. Since pH<pKa, the amine end is protonated and it will remain NH3+ (If deprotonated, it will be NH2). For the carbonyl end, the pKa will always be 3. Since pH>pKa, the carbonyl end is deprotonated and it will remain O- (If protonated it will be OH).
-
2Alter functional groups based on specific pH. Only acidic and basic side chains will be altered as well as the polar amino acids Cysteine and Tyrosine. Refer to the table in part 3, step 3.
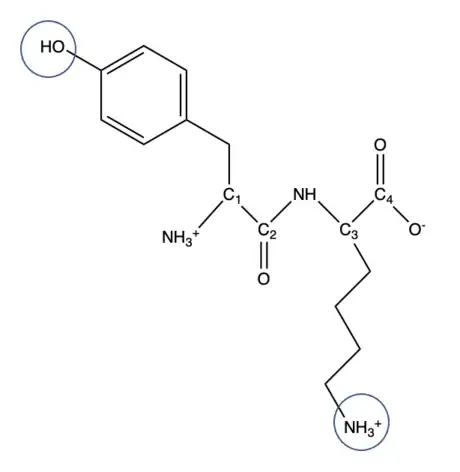
Pka of Tyr=10.07, the pH<pKa so it will be protonated and remain OH (If deprotonated it will be O-). Pka of Lys=10.79, the pH<pKa, it is protonated so it will be NH3+ (If deprotonated it will be NH2).
Finally, you have completed a drawing of a dipeptide at a specific pH.