Doravirine
Doravirine, sold under the brand name Pifeltro, is a non-nucleoside reverse transcriptase inhibitor medication developed by Merck & Co. for use in the treatment of HIV/AIDS.
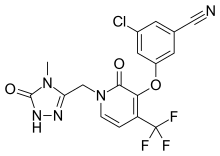 | |
| Clinical data | |
|---|---|
| Trade names | Pifeltro |
| Other names | MK-1439 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618048 |
| License data |
|
| Routes of administration | By mouth[1][2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.234.454 |
| Chemical and physical data | |
| Formula | C17H11ClF3N5O3 |
| Molar mass | 425.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Doravirine was approved for medical use in the United States in August 2018.[7]
Doravirine is available in fixed-dose combinations with other HIV drugs such as doravirine/lamivudine/tenofovir (sold under the brand name Delstrigo).[8]
References
- "Pifeltro- doravirine tablet, film coated". DailyMed. 10 October 2019. Retrieved 22 September 2020.
- Collins S, Horn T. "The Antiretroviral Pipeline" (PDF). Pipeline Report. p. 10. Archived from the original (PDF) on 11 March 2016. Retrieved 6 December 2015.
- "PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION : PIFELTRO" (PDF). Pdf.hres.ca. Retrieved 5 June 2022.
- "Drug and medical device highlights 2018: Helping you maintain and improve your health". Health Canada. 14 October 2020. Retrieved 17 April 2024.
- "Pifeltro 100 mg film-coated tablets - Summary of Product Characteristics (SmPC)". Medicines.org.uk. Retrieved 1 October 2020.
- "Pifeltro EPAR". European Medicines Agency (EMA). Retrieved 1 October 2020.
- "Drug Approval Package: Pifeltro (doravirine)". U.S. Food and Drug Administration (FDA). 9 October 2018. Retrieved 22 September 2020.
- "Delstrigo EPAR". European Medicines Agency (EMA). 4 August 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.