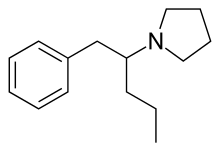
Prolintane
Prolintane (Catovit, Katovit, Promotil, Villescon) is a stimulant[2] and norepinephrine-dopamine reuptake inhibitor developed in the 1950s.[3] Being an amphetamine derivative, it is closely related in chemical structure to other drugs such as pyrovalerone, MDPV, and propylhexedrine and it has a similar mechanism of action.[4] Many cases of prolintane abuse have been reported.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, intranasal, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.007.077 |
| Chemical and physical data | |
| Formula | C15H23N |
| Molar mass | 217.356 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 133 °C (271 °F) |
| Boiling point | 153 °C (307 °F) |
| |
| |
| | |
Under the trade-name "Katovit", prolintane was commercialized by the Spanish pharmaceutical company, FHER. Katovit was sold until 2001, and was most often used by students and workers as a stimulant to provide energy, promote alertness and concentration.
See also
- α-PVP (β-ketone-prolintane, prolintanone)
- Methylenedioxypyrovalerone (MDPV)
- Pyrovalerone (Centroton, Thymergix)
- Phenylpropylaminopentane
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- Hollister LE, Gillespie HK (March–April 1970). "A new stimulant, prolintane hydrochloride, compared with dextroamphetamine in fatigued volunteers". The Journal of Clinical Pharmacology and the Journal of New Drugs. 10 (2): 103–9. doi:10.1177/009127007001000205. PMID 4392006.
- GB Patent 807835
- Nicholson AN, Stone BM, Jones MM (November 1980). "Wakefullness and reduced rapid eye movement sleep: studies with prolintane and pemoline". British Journal of Clinical Pharmacology. 10 (5): 465–72. doi:10.1111/j.1365-2125.1980.tb01790.x. PMC 1430138. PMID 7437258.
- Kyle PB, Daley WP (September 2007). "Domestic abuse of the European rave drug prolintane". Journal of Analytical Toxicology. 31 (7): 415–8. doi:10.1093/jat/31.7.415. PMID 17725890.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.