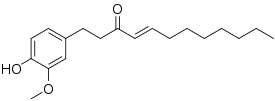
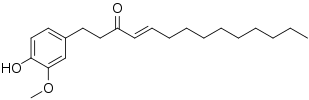
Shogaol
Shogaols are pungent constituents of ginger similar in chemical structure to gingerol. The most common of the group is [6]-shogaol. Like zingerone, it is produced when ginger is dried or cooked.[2] Moreover, shogaol (and gingerol) are converted to other constituents when heat is applied over time, which is why ginger loses its spiciness as it is cooked.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(4E)-1-(4-Hydroxy-3-methoxyphenyl)dec-4-en-3-one | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C17H24O3 | |
| Molar mass | 276.376 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Shogaol | |
|---|---|
| Heat | Very hot (chemical) |
| Scoville scale | 160,000[1] SHU |
The name shogaol is derived from the Japanese name for ginger (生姜、shōga).
Shogaol is rated 160,000 SHU on the Scoville scale.[1] When compared to other pungent compounds, shogaol is moderately more pungent than piperine, but less than capsaicin.
| Compound | Scoville Heat Units (SHU) |
|---|---|
| Capsaicin | 16,000,000[3] |
| [6]-Shogaol | 160,000 |
| Piperine | 100,000 |
| [6]-Gingerol | 60,000 |
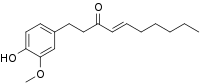
Shogaols group
[4]-Shogaol, [8]-shogaol, [10]-shogaol, and [12]-shogaol (all found in ginger) together constitute the group shogaols. There also exist in ginger cultivars methylated shogaols: methyl [6]-shogaol and methyl [8]-shogaol, respectively.[4]
Shogaols are artifacts formed during storage or through excess heat, probably created by a dehydration reaction of the gingerols. The ratio of shogaols to gingerols sometimes is taken as an indication of product quality.[5]
Synthesis
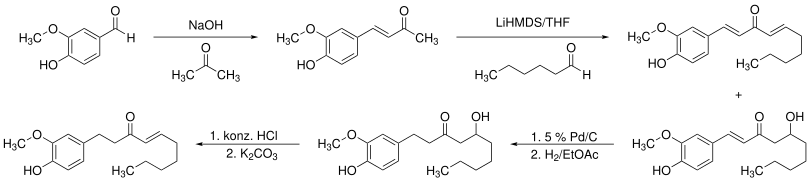
A possible synthesis starts with a Claisen condensation of vanillin and acetone, producing dehydrozingerone. Afterwards the product reacts in an aldol condensation with hexanal in tetrahydrofurane to 6-dehydroshogaol and 6-dehydrogingerol. Latter can be hydrogenated to [6]-gingerol by a catalyst. In the last step hydrochloric acid is added to get the desired [6]-shogaol.[6]
References
- Compton, Richard G.; Batchelor-McAuley, Christopher; Ngamchuea, Kamonwad; Chaisiwamongkhol, Korbua (2016-10-31). "Electrochemical detection and quantification of gingerol species in ginger (Zingiber officinale) using multiwalled carbon nanotube modified electrodes". Analyst. 141 (22): 6321–6328. Bibcode:2016Ana...141.6321C. doi:10.1039/C6AN02254E. ISSN 1364-5528. PMID 27774555. S2CID 40241982.
- Harold McGee (2004). On Food and Cooking: The Science and Lore of the Kitchen (2nd ed.). New York: Scribner. pp. 425–426.
- Govindarajan, Sathyanarayana (1991). "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
- "Analysis of Chemical Properties of Edible and Medicinal Ginger by Metabolomics Approach : Table 1". Retrieved 3 December 2016.
- NSF International Determination of Gingerols and Shogaols in Zingiber officinale rhizome and powdered extract by High-Performance Liquid Chromatography
- Hung-Cheng Shih; et al. (March 2014). "Synthesis of Analogues of Gingerol and Shogaol, the Active Pungent Principles from the Rhizomes of Zingiber officinale and Evaluation of Their Anti-Platelet Aggregation Effects". International Journal of Molecular Sciences. 15 (3): 3926–3951. doi:10.3390/ijms15033926. PMC 3975376. PMID 24599082.