Lithium tantalate
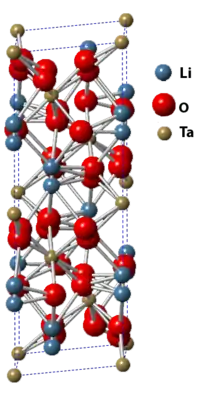
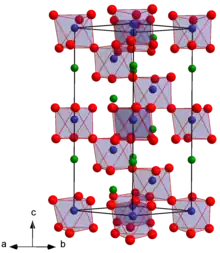
Lithium tantalate is the inorganic compound with the formula LiTaO3. It is a white, diamagnetic, water-insoluble salt. The compound has the perovskite structure. It has optical, piezoelectric, and pyroelectric properties that make it valuable for nonlinear optics, passive infrared sensors such as motion detectors, terahertz generation and detection, surface acoustic wave applications, cell phones. Considerable information is available from commercial sources about this material.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lithium tantalate | |
| Other names
Lithium Metatantalate | |
| Identifiers | |
| ECHA InfoCard | 100.031.584 |
PubChem CID |
|
| RTECS number |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| LiTaO3 | |
| Molar mass | 235.887 g/mol |
| Density | 7.46 g/cm3, solid |
| Melting point | 1,650 °C (3,000 °F; 1,920 K) |
| Insoluble in water | |
| Structure | |
| Space group R3c | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Acute Toxicity: Oral, Inhalation, Dermal |
| Safety data sheet (SDS) | http://www.samaterials.com/pdf/Lithium-Tantalate-Wafers-(LiTaO3-Wafers)-sds.pdf |
| Related compounds | |
Other anions |
LiNbO3 |
| Supplementary data page | |
| Lithium tantalate (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Applications and research
Lithium tantalate is a standard detector element in infrared spectrophotometers.[2]
Pyroelectric fusion has been demonstrated using a lithium tantalate crystal producing a large enough charge to generate and accelerate a beam of deuterium nuclei into a deuterated target resulting in the production of a small flux of helium-3 and neutrons through nuclear fusion without extreme heat or pressure.[3]
A difference between positively and negatively charged parts of pyroelectric LiTaO3 crystals was observed when water freezes to them.[4]
See also
- Lithium tantalate (data page)
References
- Abrahams, S.C; Bernstein, J.L (1967). "Ferroelectric lithium tantalate—1. Single crystal X-ray diffraction study at 24°C". Journal of Physics and Chemistry of Solids. 28 (9): 1685. Bibcode:1967JPCS...28.1685A. doi:10.1016/0022-3697(67)90142-4.
- "Application note: Infrared Spectroscopy" (PDF).
- B. Naranjo, J.K. Gimzewski & S. Putterman (2005). "Observation of nuclear fusion driven by a pyroelectric crystal". Nature. 434 (7037): 1115–1117. Bibcode:2005Natur.434.1115N. doi:10.1038/nature03575. PMID 15858570. S2CID 4407334.
- D. Ehre; E. Lavert; M. Lahav; I. Lubomirsky (2010). "Water Freezes Differently on Positively and Negatively Charged Surfaces of Pyroelectric Materials". Science. 327 (5966): 672–675. Bibcode:2010Sci...327..672E. doi:10.1126/science.1178085. PMID 20133568. S2CID 206522004.